The way to diagnose and manage asthma has changed with FeNO testing.

NICE guidelines and FeNO:
Key points
A FeNO level of 50 parts per billion (ppb) or more suggests significant eosinophilic airway inflammation and supports an asthma diagnosis.
A FeNO level of 35 ppb or more indicates eosinophilic airway inflammation, supporting asthma diagnosis.
FeNO testing should be used alongside spirometry and bronchodilator reversibility (BDR) testing to confirm or rule out asthma.
FeNO variability can help guide treatment adjustments, particularly in determining the effectiveness of inhaled corticosteroids (ICS).
QOF
The Quality and Outcomes Framework (QOF) now incorporates FeNO testing as an essential part of asthma care. This reflects NICE NG245’s emphasis on objective testing to improve diagnostic accuracy and treatment efficacy.
Key QOF Indicators Include:
Using FeNO tests to confirm asthma in children and adults with suspected airway inflammation.
Combining FeNO results with spirometry and clinical assessments for a holistic approach to asthma diagnosis.
Monitoring FeNO variability to assess response to treatment and guide changes in therapy.
These indicators aim to reduce misdiagnoses, enhance personalised care, and promote evidence-based practices across healthcare systems.

Why the change?
Asthma is a complex condition with symptoms that overlap with other respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and chronic cough. Traditional diagnostic methods often rely on subjective assessments or spirometry alone, leading to potential misdiagnoses or delayed treatment. The updated NICE NG245 guidance addresses these challenges by prioritising FeNO testing.
Key Reasons for the Change:
Improved Diagnostic Precision:
FeNO testing directly measures eosinophilic inflammation, a hallmark of asthma.
By providing clear FeNO levels, it aids in distinguishing asthma from non-asthmatic conditions.
Enhanced Treatment Decisions:
FeNO and inhaled corticosteroids: FeNO results help determine whether patients need ICS treatment and monitor their effectiveness over time.
High FeNO levels indicate uncontrolled inflammation, necessitating a review of therapy.
Optimised Outcomes:
FeNO testing ensures patients receive appropriate treatment, reducing the risks of exacerbations and improving quality of life.
By including FeNO for asthma diagnosis, the NICE guideline offers a pathway for timely, accurate care.


Diagnostic algorithms
NICE Diagnostic Algorithm for Asthma:
Initial Clinical Assessment:
Document symptoms, including wheezing, breathlessness, chest tightness, and cough.
Identify potential triggers, such as allergens, exercise, or occupational exposures.
Objective Testing:
FeNO Testing:
Adults: A FeNO level of 50 ppb or more indicates eosinophilic inflammation.
Children (5–16 years): A FeNO level of 35 ppb or more suggests eosinophilic asthma.
FeNO levels below these thresholds do not exclude asthma but warrant further testing.
Spirometry:
Measures airflow obstruction. A reduced FEV1/FVC ratio supports an asthma diagnosis.
Bronchodilator Reversibility (BDR):
Assesses reversibility of airway obstruction after bronchodilator use.
Integrated Diagnosis:
Combine FeNO results with spirometry, BDR findings, and clinical history to confirm or rule out asthma.
Reassess with FeNO and spirometry if results are inconclusive.
Reassess and Monitor:
Use FeNO variability to guide treatment adjustments and evaluate long-term control.
FeNO in Asthma Management
FeNO testing is a cornerstone of modern asthma management, offering actionable insights into airway inflammation and guiding personalised treatment strategies.
Applications in Asthma Management:
Guiding ICS Therapy:
High FeNO levels suggest a need for increased corticosteroid doses, while low levels may indicate successful inflammation control.
Monitoring Disease Progression:
Regular FeNO testing ensures inflammation levels are managed effectively, reducing exacerbations.
Regular FeNO testing ensures inflammation levels are managed effectively, reducing exacerbations.
Identifies inflammation caused by workplace exposures and guides mitigation strategies.
Tracks changes in airway inflammation over time, enabling proactive adjustments to treatment plans.
By integrating FeNO for asthma monitoring, healthcare providers can ensure optimal patient outcomes.
Download NICE guidelines
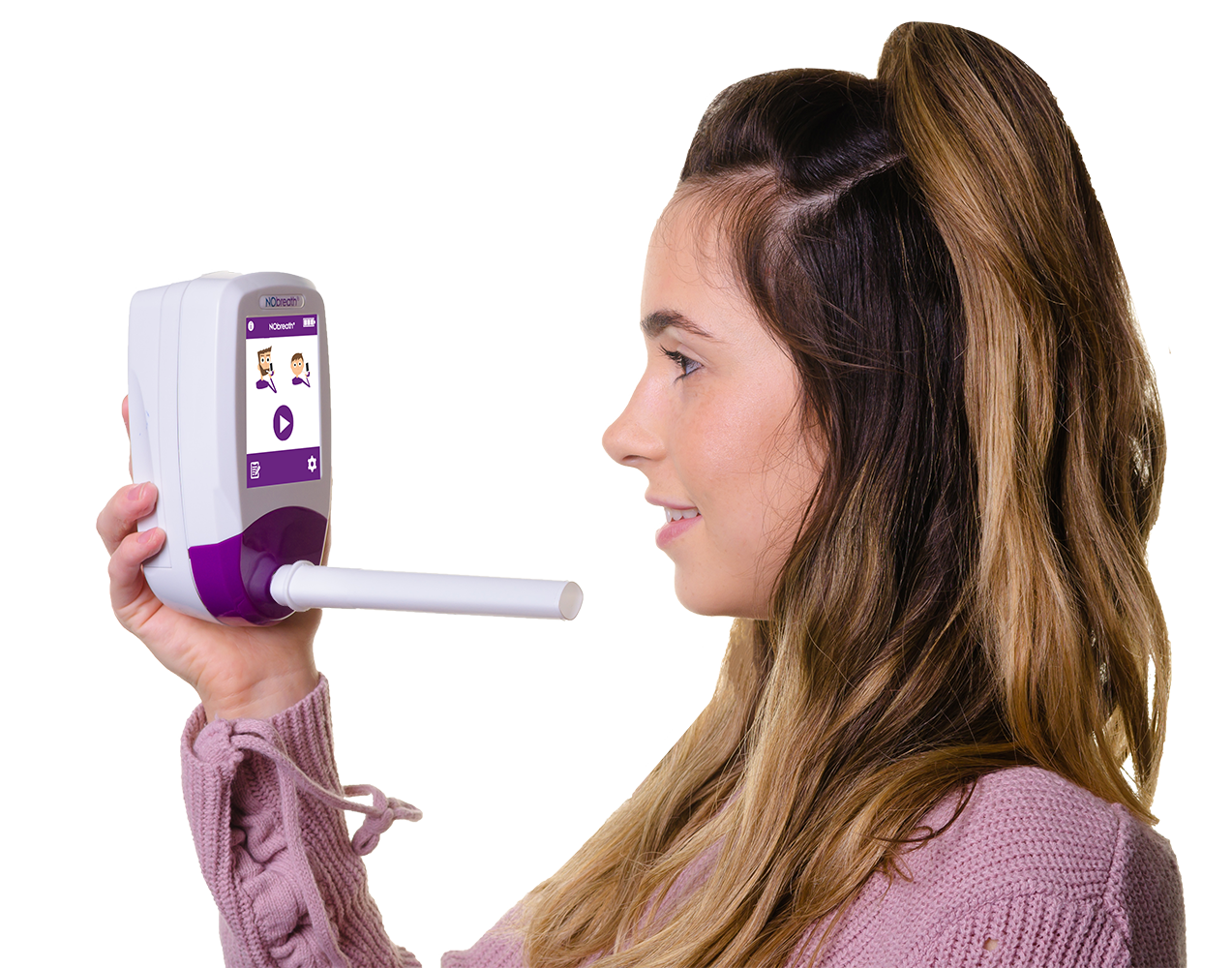
NObreath® FeNO testing device to diagnose and monitor asthma
This innovative, yet widely used in primary care tool, provides accurate, non-invasive FeNO measurements, making it a valuable resource for clinicians diagnosing and managing asthma.
Why Choose NObreath®?
High Precision:
Delivers accurate FeNO measurements to identify eosinophilic inflammation, supporting asthma diagnosis and treatment decisions. Evaluated against Chemiluminescence Method, the gold standard for FeNO measurement. (reference chemiluminescence evaluation Doc)
User-Friendly Design:
Compact, portable, and easy to use, suitable for GP surgeries, CDC’s, diagnostic hubs, asthma clinics and busy acute settings.
Cost-Effective:
Flexible capital cost with low entry yearly rental option available for £695 including servicing costs.
Dedicated Primary care equipment offers to allow easier financial access to the testing device.
Lower cost of consumables when compared to other FeNO testing system.
5 Years Warranty on monitor and sensor.
Real-Time Results:
Enhanced Monitoring:
Tracks FeNO variability to assess the effectiveness of treatments like ICS.
Unique Features of the NObreath® FeNO monitor:
Fully compliant with FeNO guidelines for asthma care.
Supports personalised treatment plans by measuring FeNO levels in asthma, FeNO in allergic asthma, and FeNO in occupational asthma.
References
We have used facts and figures from NICE and other reputable sources to compile the content on this web page about the NICE guidelines. These are referenced in the bibliography below.
NICE QOF Indicators: Objective testing in asthma https://www.nice.org.uk/indicators/ind272-asthma-objective-tests/chapter/indicator
NICE Asthma guidance resources https://www.nice.org.uk/guidance/ng245/resources
Bedfont® NObreath® FeNO FeNo testing device: NObreath product details, user manual and technical specifications https://resources.bedfont.com/nobreath-resources/
Chemiluminescence validation study 2011 – Kapande et al. – Comparative Repeatability of Two Handheld Fractional Exhaled Nitric Oxide Monitors

