
In Secondary Care.
A cost effective FeNO solution for delivering airway inflammation testing without high running costs or contract requirements

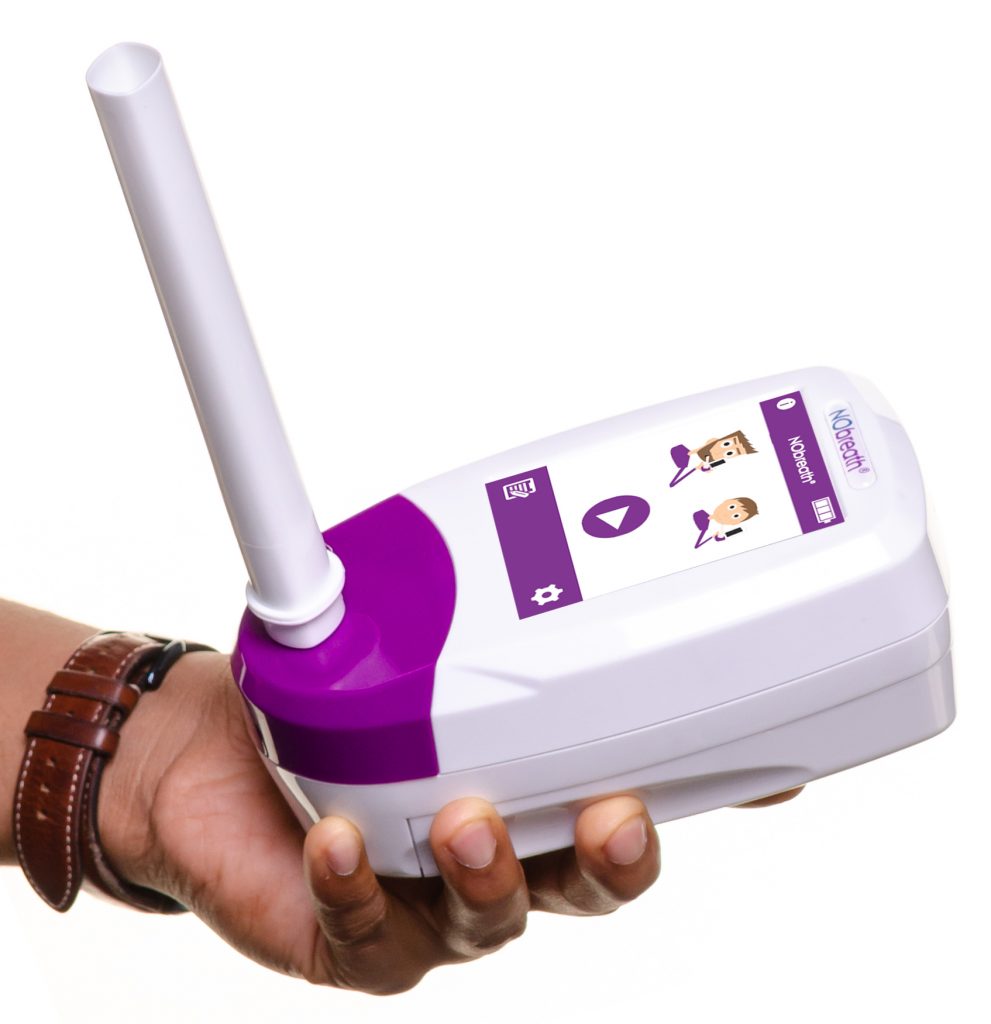
FeNO testing that costs just £3.70 per patient, supporting efficient delivery at scale
FeNO testing has traditionally been costly for many respiratory services across the UK, particularly in secondary care where patient volumes are higher and more complex assessments are required. The NObreath® FeNO monitor provides a cost effective way to deliver fractional exhaled nitric oxide testing, reducing the average running cost to around three pounds and seventy pence per patient. This allows hospital teams to incorporate FeNO more easily into asthma diagnosis and ongoing management pathways.
Calculate Your FeNO Savings with NObreath
NObreath uses single patient mouthpieces that can be reused multiple times by the same individual. This helps minimise consumable usage while maintaining consistent, high quality FeNO measurements. The reduced running cost and straightforward workflow make NObreath a practical and sustainable option for respiratory departments, severe asthma services and diagnostic hubs seeking to expand access to airway inflammation testing.
How much can your department save in one year?
Compare the running costs of your current device against NObreath® to find out how much your department can save annually on FeNO testing.
Key Features of NObreath for Secondary Care
Perform test after test. Without limitations.
The Bedfont® NObreath® FeNO monitor places no restrictions on the number of FeNO tests you can perform, allowing continuous airway inflammation testing without usage limits. The device does not need to be replaced after a set number of uses, making it one of the most cost-effective FeNO testing solutions for secondary care and respiratory services. Its electrochemical sensor is designed to operate reliably for five years when maintained with regular servicing, supporting accurate FeNO measurement for asthma diagnosis and ongoing asthma management in line with UK clinical pathways and NICE NG245 guidance.

Proven technology.
For over 10 years.
The Bedfont® NObreath® FeNO monitor uses a proven electrochemical sensor, and its accuracy is validated against chemiluminescence, the recognised gold standard for FeNO measurement.1 The technology has been assessed in multiple clinical studies and case reports, demonstrating reliable FeNO testing across a wide range of primary and secondary care respiratory settings. NObreath® was also included in a recent NICE feasibility study that informed updates to the latest asthma guidelines (NICE NG245), further reinforcing its clinical credibility for supporting asthma diagnosis, airway inflammation monitoring and long-term asthma management.
Single patient, multi-use mouthpieces
A patient can be tested multiple times using the same NObreath® mouthpiece, which helps reduce FeNO testing costs in busy clinical environments. Each mouthpiece contains a one way valve and a high quality viral and bacterial filter to reduce the risk of cross infection between patients and to support strong infection control standards in both primary and secondary care respiratory settings. The multi use design also helps clinicians achieve consistent and reliable FeNO measurements for asthma diagnosis and ongoing airway inflammation monitoring..

Made with an anti-microbial body
NObreath® is manufactured with SteriTouch®, an antimicrobial additive that is incorporated into the exterior plastic casing. This helps kill bacteria on contact and reduces the risk of cross infection during routine use in clinical environments. The antimicrobial surface provides an additional layer of protection for respiratory teams carrying out regular FeNO testing for asthma diagnosis and airway inflammation monitoring.
Want to see NObreath in action?
Watch the Demonstration VideoDownloads
Clinical Papers
- 2014 – L Bjermer et al. – Current Evidence and future research needs for FeNO
- 2013 – Saito et al. -Domiciliary diurin l variation of fractional exhaled nitirc oxide for asthma control
- 2012 – Takeno et al. – Measurements of Nasal FENO
- 2012 – Jedrychowski et al. – Maternal Allery as a Potential Source of Variability of ENO in Children
- 2011 – Kapande et al. – Comparative Repeatability of Two Handheld Fractional Exhaled Nitric Oxide Monitors – NObreath
- 2010 – Pisi et al. – Measurement of FENO by a new portable device the NObreath
- 2010 – Antus et al. – Assessment of exhaeld NO by a new hand-held device the NObreath
PPQ/PAQ Forms
For hospital procurement teams, the NObreath® pre-purchase questionnaire is available on request. Please contact Intermedical on 01732 522444.
Data Sheets
- NObreath Datasheet
- NObreath FeNO Interpretation Chart
- NObreath Catalogue
- NObreath – Version 1 and 2 comparison
NICE. National Institute for Health and Care Excellence

